Research
In short, my research focusses on the investigation of mechanical, thermal and adsorption properties of metal-organic frameworks for new applications such as nano-mechanical devices for energy storage and selective adsorbers for sensing of various gasses and separation of gas mixtures. To this end, force fields are derived from ab initio input data using our in-house developed code QuickFF and applied in a wide range of molecular simulations. Finally, the output is used for developping advanced thermodynamic models that allow to apply realistic environmental conditions. The underlying workflow for this research is summarized in the following figure: 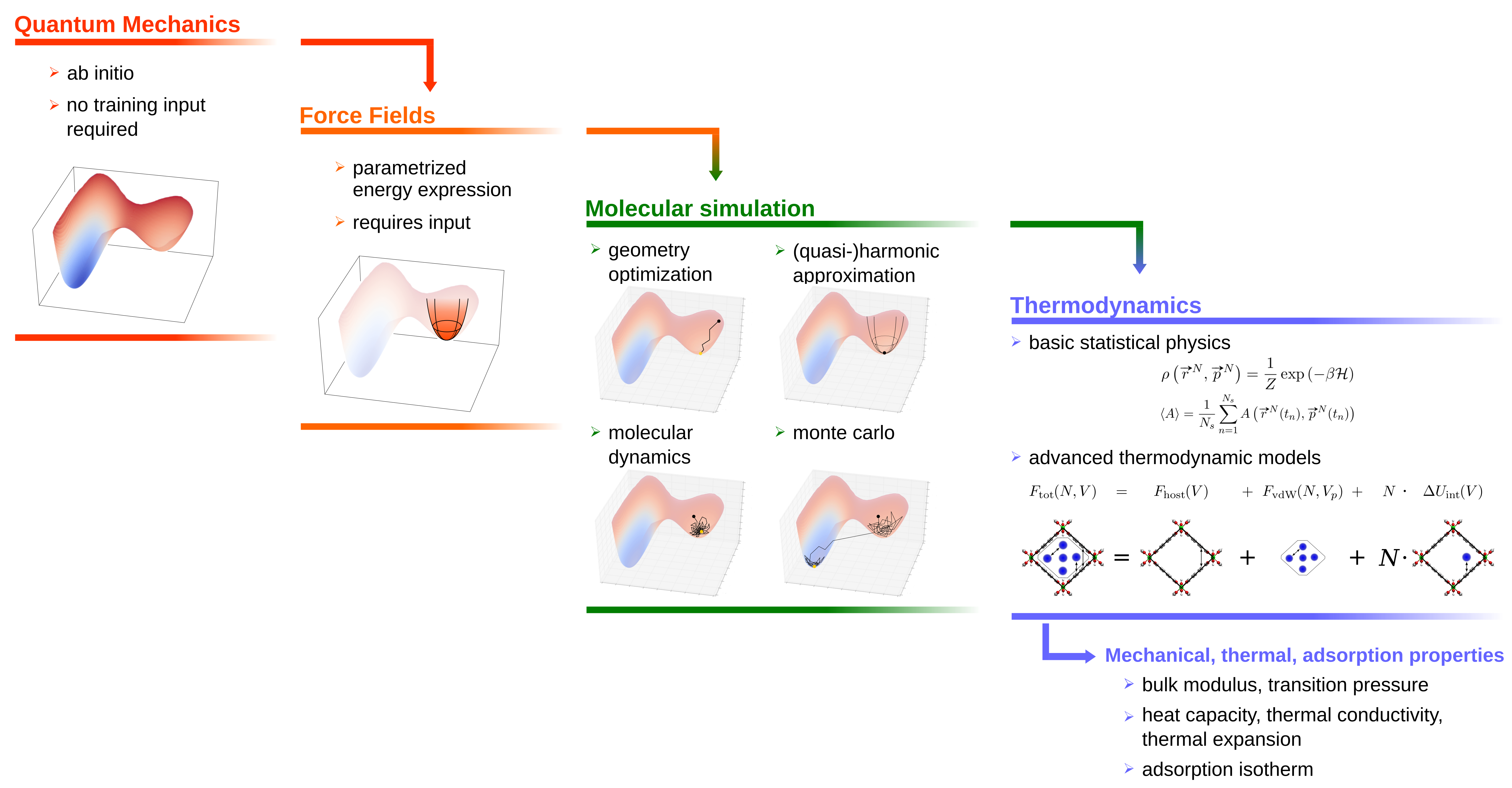 Below, a more detailled description of the Methodolgy and Applications of my research is provided:
Below, a more detailled description of the Methodolgy and Applications of my research is provided:
Methodology
- Force field development
I develop accurate force fields that mimic the quantum mechanical behaviour of the system. This is achieved by fitting force fields to reproduce the ab initio geometry and Hessian in equilibrium. The research resulted in a python package (QuickFF) to quickly derive accurate force fields from ab initio input. More information on how to install and use QuickFF can be found here. - Advanced Molecular Simulations
The force fields are then used in Molecular Dynamics (MD), Monte Carlo (MC) as well as classical Density Functional Theory (cDFT) simulations to efficiently sample the phase space of the system. These can involve simple geometry optimizations followed by a (quasi-)harmonic approximation. However, depending on the molecular properties we are interested in, more advance ensembles and techniques may be applied such as thermodynamic integration, umbrella sampling, metadynamics, variationally enhanced sampling or replica exchange. Finally, cDFT simulations are used to compute adsorption properties of nanoporous materials in a quickff yet accurate way. - Thermodynamics and Statistical Physics
Once we dispose of trajectories that efficiently sample the phase space, we can use them to compute several thermodynamic properties such as thermal energy, entropy, heat capacity, equilibrium volume, ... In many cases, we are interested in the dependency of the free energy to a given degree of freedom. This can either involve a collective variable describing a certain microscopic degree of freedom, or a thermodynamic variable such as the volume. Unfortunately, calculating the free energy (and therefore the entropy) from molecular dynamics simulations is not straightforward and requires the enchanced sampling techniques mentioned in the previous paragraph. However, by applying either a straightforward histogram analysis or its more advanced variant WHAM, we are able to extract such free energy profiles from molecular simulations. As such, we gain access to a wide variaty of thermodynamic properties such as bulk modulus, phase transition pressures, thermal expansion coefficient, adsorption uptake as well as kinetic properties such as the rate constant of a chemical reaction or physical process (e.g. diffusion). The methodologies to construct and manipulate free energy profiles, including adequate error estimation, are implemented in our in-house software package ThermoLIB. Finally, I also develop (semi-)analytical models that can easily transform the computed input data between different ensembles. These models contain parameters such as accessible pore volume and adsorption energy that we extract from molecular simulations. As such, we are able to constraint the system to satisfy realistic experimental conditions and extract the relevant thermodynamic properties.
Applications
Most of my research is applied to Metal-Organic Frameworks (MOFs), which are hybrid materials consisting of inorganic metal clusters connected to each other by means of organic linkers, resulting in periodic frameworks (crystals) with pores of several nanometers (nanoporous). Some of these materials are also known to exhibit flexible behaviour when exposed to various triggers such as temperature, pressure and adsorption of gasses. As such, it was discovered that they are capable of transforming between various phases accompanied by substantial changes in the unit cell volume (up to 40%) while retaining their structural integrity. This phenomenon is commonly denoted as breathing. Furthermore, since the building blocks are held together by rather weak interactions (dispersive interactions, pi-pi stacking, hydrogen bonds,...), their response towards these stimuli can be especially uncommon compared to traditional microporous materials. Various counterintuitive phenomena have been discoverd such as negative linear compression (the system expands along one or more directions upon hydrostatic compression), negative thermal expansion (materials contract upon heating) and negative gas adsorption (the material release adsorbed gas from its pores at some point when the vapor pressure of the surroundings increases). To describe these phenomena on a large scale, one requires (1) accurate force fields to generate the microscopic data (2) efficient sampling protocals to scan all relevant parts of the potential energy surface (PES) and (3) advanced thermodynamic models to impose realistic working conditions. Due to their nanoporosity, MOFs have some very attractive applications such as detection, separation and detection of gasses, catalysis of chemical reactions, nanosprings and nano shock absorbers, drug delivery systems and more. My research specifically focusses on the thermodynamic characterization of MOFs with respect to their mechanical (bulk modulus, transition pressure of phase transition), thermal (thermal expansion coefficient, heat capacity) and adsorption (adsorption uptake, affinity, selectivity) properties to allow for an computationally-aided design of high-performance materials for new applications.
Teaching
I am the responsible lecturer for the following courses taught at Ghent University:
- Modelling and Engineering of Nanoscale Materials
- Kwantummechanica I (Quantum Mechanics I)
- Natuurkunde III (Thermodynamics and Statistical Physics)
Furthermore, I am a co-lecturer for the following courses taught at Ghent University:
- Statistische Fysica en Moleculaire Structuur (Statistical Physics and Introductory Quantum Mechanics)