Towards accurate prediction of alkene diffusivities in metal substituted aluminophosphates during MTO conversion
Towards accurate prediction of alkene diffusivities in metal substituted aluminophosphates during MTO conversion
Promotor(en): V. Van Speybroeck, L. Vanduyfhuys /25568 / Nanoporous materialsBackground and problem
The methanol to olefins (MTO) synthesis on acid zeolite catalysts received a lot of attention during the last decades as a more sustainable alternative for the production of base chemicals. The MTO mechanism is very complex and still not fully understood up to date. Both aromatic and aliphatic hydrocarbon pool (HP) species inside the catalyst pores were found to assist in the conversion of methanol. The ultimate product distribution is determined by a variety of factors such as the stability of intermediates, catalytic reactivity and transport phenomena. [1,2] Especially, the role of alkane and alkene diffusion on the product selectivity remains unanswered. The ease of these diffusion paths relies on the pore topology and channel dimensions of the framework, but also on the occupation of the zeolite pores by the HP species which may block the channel system and restrict diffusion. [3] Recently, it was shown that the presence of acid sites in zeolite H-SAPO-34 may have a considerable effect on the diffusivities of small alkenes and alkanes. [4] The precise influence of the acid site strength and density on the other hand still remains a missing link.
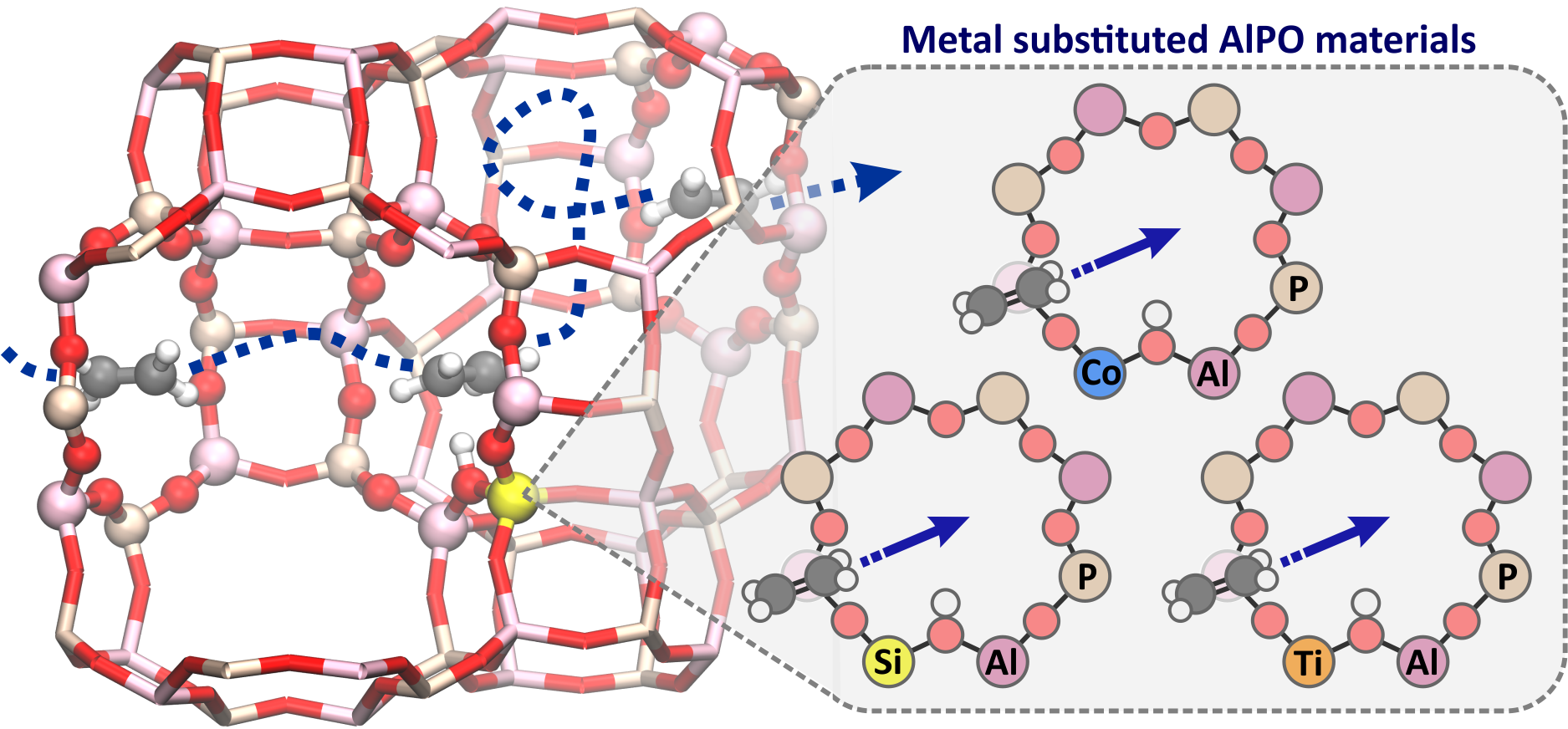
Figure 1. Schematic representation of a diffusion pathway in metal substituted aluminophosphate materials.
Zeolites are structurally ordered nanoporous materials possessing channels and pores of molecular dimensions, which makes them very tractable for catalytic or separation purposes. The function of these materials relies to a large extent on the diffusion of small molecules through the channel and pore system as schematically shown in Figure 1. Molecular simulations can contribute to attaining a proper molecular level understanding of the diffusion paths in these materials. The diffusion phenomenon takes place at larger length and time scales and is therefore typically studied using classical force fields. However, light olefin diffusion in a complex molecular environment might be more accurately described using a first-principle description. [4] A proper theoretical understanding of diffusion is mandatory for designing zeolites with a higher light olefin selectivity in the MTO process or with applications for product separation.
Goal
In the framework of this master thesis, we want to investigate how the acid site strength and distribution in the zeolite framework can be altered to promote the diffusion of light olefins. To this end, you will study the diffusion of small hydrocarbons (propene, propane,…) in a set of metal substituted aluminophosphate zeolites to properly assess the influence of the varying acid site strength and acid site distribution on the diffusion rates. [5] Furthermore, we will consider diffusion in a pore system which is representative for the actual MTO environment. Specific case studies in which the channel system is (partially) blocked by methanol reactant and/or HP species will be taken into account. To properly model the transport phenomenon at these conditions, first-principle MD simulations and rare event sampling techniques will be performed. The obtained insights into the diffusion properties obtained from the MD simulations will be applied for constructing a model to predict diffusivities which can be compared with experimentally measured data. Ultimately, the insight into the diffusion behaviour of small hydrocarbons may assist the efforts for increasing the MTO product selectivity.
The Center for Molecular Modeling has ample experience in modeling of zeolite catalysis with advanced simulation techniques. The proposed topic is situated in a very active research field. You will be actively coached to get acquainted with the multitude of available techniques to tackle. You will be actively involved in the running collaboration with prof. Unni Olsbye (University of Oslo) on this topic.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsZeolite catalysis, Diffusion, Methanol-to-olefinsReferences
[1] P. Ferri et al., ACS Catal. 9 (2019) 11542-11551.
[2] M. Gao et al., Nat. Commun. 11 (2020) 3641.
[3] Y. Shen et al. ACS Catal. 8 (2018) 11042-11053.
[4] P. Cnudde et al., Angew. Chem. Int. Ed. (2021).
[5] M. Morten et al., ChemPhysChem 19 (2018) 484-495.
