Zirconium sites for epoxide rearrangement: from zeolites to MOFs
Zirconium sites for epoxide rearrangement: from zeolites to MOFs
Promotor(en): V. Van Speybroeck /17NANO04 / Nanoporous materialsZeolites and metal-organic frameworks (MOFs) (Figure 1) are a well-established family of nanoporous materials which can be used in heterogeneous catalysis and are of vital importance for the chemical industries. They provide crucial features for industrial applications, such as high surface area, uniform porosity, inter-connected pore/channel system, accessible pore volume, high adsorption capacity, ion-exchange ability, enhanced catalytic activity, and shape/size selectivity. Even though zeolites and MOFs share all these qualities, the chemical properties of both classes of these materials are completely distinctive. Zeolites are constructed from purely inorganic building blocks, typically Si tetrahedra surrounded by oxygen atoms. They contain both Brønsted and Lewis acid sites which make them catalytically active. Replacing single silicon framework atoms by other metals as for instance zirconium creates isolated Lewis acid sites.
In contrast to zeolites, MOFs constitute a new class of porous materials that exhibit a truly hybrid character as they are made up of both inorganic and organic moieties. In some exceptional cases MOFs can reach the chemical stability of zeolites. One of the most widely investigated MOFs from the plethora of known frameworks is the Zr(IV)-terephthalate MOF designated as UiO-66. This thermally stable MOF is built up not from single atoms but from nodes in the structure that are formed by oxygen-bridged Zr6 octahedra. The hexa Zr cluster can be dehydroxylated under specific conditions leaving open metal sites which are available for catalysis. At elevated temperatures and low pressure the Zr core gets dehydroxylated and rearranged, creating coordinated vacancies (defects) which results in a presence of Lewis acid sites [1].
Metal zeolite Zr-beta with well-defined single isolated sites and UiO-66 have been reported to be excellent and highly selective catalysts for Lewis acid catalyzed reactions such as epoxide rearrangement [2].
Rearrangement of β-pinene epoxide (1) into the natural terpenoid myrantal is of potential industrial interest, as myrantal (2) is a relevant compound for the fragrance industry. For the transformation of β-pinene epoxide into this fine chemical product, a solid catalyst is preferred to soluble acids since the former can be easily separated and reused. Heterogeneous catalysis is one of the disciplines that have introduced the most breakthroughs in the field of chemical reactivity. Until recently, most of the progresses were made on the basis of chemical intuition and analogies. However, the major development in this field will come through a better understanding of the catalytic mechanism at a molecular level.
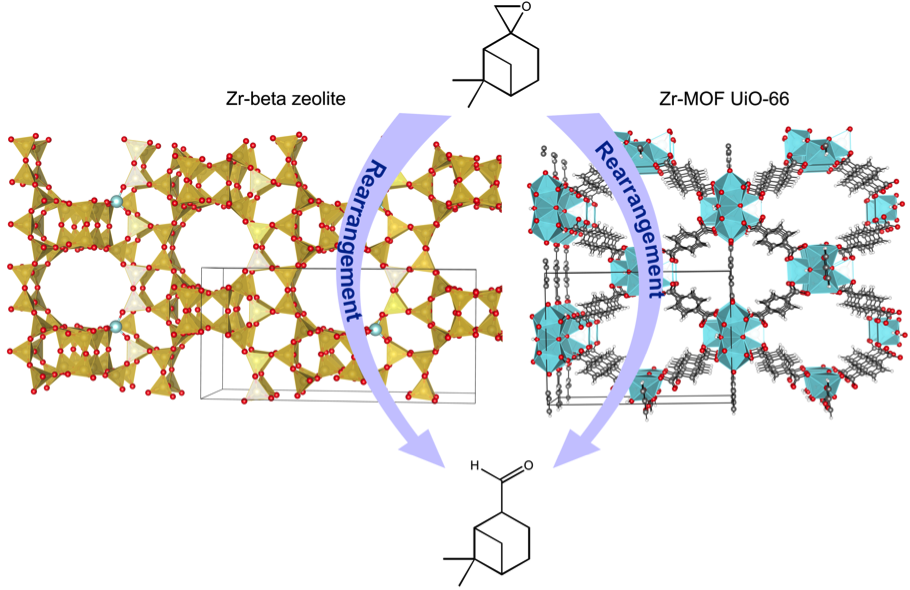
Figure 1: Structure of Zr-beta zeolite (left) and Zr-MOF UiO-66 (right). Rearrangement of β-pinene epoxide (1) into myrantal (2).Goal
The objective of this master thesis project is to compare the activity and selectivity of two Lewis acid catalysts, namely Zr-beta zeolite and the Zr-MOF UiO-66 for the transformation of β-pinene epoxide into myrantal, a relevant compound for the fragrance industry. Despite the number of experimental studies on zeolites, molecular insights into the location of Zr in zeolite beta are missing [1, 2]. In this view, the first task of this thesis will be to determine the most stable position of Zr in zeolite beta framework as well as the nature of the defect sites in UiO-66. In a second step the reaction mechanisms on both catalysts will have to be unraveled going from small non-periodic models to fully periodic systems. The reaction mechanism is not straight forward as several bi-products might be formed. For this purpose, first principle chemical kinetics simulations will be performed and the full reactive profile will be calculated. Next, the techniques will be used to construct and calculate a theoretical selectivity model. The results will be compared to experimental structural, spectroscopic and kinetic data available in literature [1, 2]. The Center for Molecular Modeling has gained a lot of experience with modeling catalysis in zeolites and MOFs, and has access to vast computational resources and a broad variety of computational programs to perform such type of simulations. Intensive support will be provided during the project, which is part of a highly active research topic of the group. The proposed topic is challenging and requires technical skills, creativity and chemical insight. The results obtained will be highly relevant for further research in heterogeneous catalysis and for the theoretical understanding of defect containing materials.
Aspects
Chemical aspect: Structure determination, unraveling reaction mechanisms.
Engineering aspect: Application to the catalytic properties of nanoporous materials.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsHeterogeneous Catalysis, Zeolites, Metal-organic frameworks, epoxide rearrangementReferences
[1] A. Corma, L.M. Orozco, M. Renz, From MOFs to zeolites: zirconium sites for epoxide rearrangement, New J Chem, 37 (2013) 3496-3502.
[2] O. de la Torre, M. Renz, A. Corma, Biomass to chemicals: Rearrangement of beta-pinene epoxide into myrtanal with well-defined single-site substituted molecular sieves as reusable solid Lewis-acid catalysts, Appl Catal a-Gen, 380 (2010) 165-171.

